Whisky
Article curated by Rowena Fletcher-Wood
What’s in a name? A rose by any other name may smell as sweet, but the chemicals behind smell and taste still elude us. Even in some commonly consumed products like whisky.

There’s lots [of chemistry] going on in whisky.– Ben Challen Image credit: Public domain
What even is a whisky? The name normally refers to a cask aged grain spirit at least 40% alcohol by volume. Ben Challen, an erstwhile chemist and now whisky connoisseur at The Whisky Shop in Oxford describes it as Gaelic in origin (culture) and notes that to be called Scotch it must be distilled, matured and bottled in Scotland. The starting ingredients are simple: grain, yeast, water, and sometimes a little bit of caramel colouring, but whisky itself is not simple. Although whisky-making is regulated and made from natural ingredients, chemical changes take place during processing, creating new compounds with new chemical profiles. Only by knowing the starting compounds, the reactions they undergo, when they’ll undergo them and what will come out of them, can we really assess this process – and this is not something we know a whole lot about. Whisky-making has long been considered an art and, as such, there has been limited chemical exploration of its constituents. Some people want to preserve the mystery, but others want to illuminate it – to learn more and to make better, healthier whiskies. Some argue this could lead to the promotion of whisky-drinking, and that this is not a good thing as people might over-indulge. On the other hand, if we develop new whiskies with congeners that are less harmful to health or that give less severe hangovers, we also improve our health and productivity. Slowly, the whisky industry has started to explore its chemistry, mostly by dividing them into categories of molecules, and there are now a few things we do know about whiskies... There are three stages to whisky making: fermentation, distillation and maturation (or aging). Flavour is imparted at all three stages. Distillation is supposed to be a physical process: the separation (allowing the selection) of chemical molecules via boiling points – chemical reactions are not supposed to take place – but of course they do; and do so, at these high boiling temperatures, very fast indeed). The fermentation and maturation stages are more straightforward. Fermentation is the period of frenzied chemical activity when alcohol is produced from sugars and the bulk of whisky molecules are made. Maturation is a low temperature, slow chemical stage, where existing molecules in the whisky react together, and entities found in the wood are slowly leached out and combined into the profile. Originally, there was no chemical stage called aging – alcoholic drinks were put into barrels for storage and transport: barrels were supposed to be chemically inactive. But, since they never were, and did good rather than bad things to drinks such as whisky, barrel aging is now a deliberate chemical step. So – other than alcohol – what is in a whisky?


Esters, and lots of them
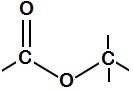
Esters are the perfumes of whisky, providing much of the flavour and nose. Esters are common molecules, named because of a special functional group – a carbon atom attached to two oxygens, one with a double bond and one with a single bond, which itself is attached to another carbon. It’s because of this bond that esters are highly volatile, easily evaporate and so end up in your nose, being detected. Esters are made from organic acids and alcohols joining together. Although ester molecules are bigger than water molecules, they evaporate more easily and so seem smelly. You will recognise some esters even if you haven't heard their chemical names, like ethyl acetate – the flavour of pear drops, or allyl hexanoate – the flavour of pineapple. The shorter chain esters tend to smell more ‘chemically’ and less like food, and it is these that evaporate off fastest during distillation and are removed with the ‘heads’ vapours, many of which are toxic. The heads are thrown away. Very short chain, toxic methyl esters either vanish as heads or get absorbed into the barrel wood during maturation. Although traces may be left, these are not in hazardous concentrations. Whisky esters are almost always ethyl (sometimes called acetyl) esters. Ethyl esters form in the reaction of ethanol (alcohol) with organic acids at elevated temperatures. Organic acids, heat and alcohol are the products of fermentation – yeast digesting sugar. Heat and alcohol will eventually kill the yeast off, but the longer it can survive, the more and the longer the organic acids it makes, so the longer the esters and more complex the whisky. Different strains of yeast give different flavours: they produce different numbers and kinds of enzymes, which catalyse different reactions and so produce different esters. They also contain different strains of bacteria, which make different lactic acids and are more or less threatening to the yeast, changing how long it survives and how many fatty acids it makes[1]. Small differences in conditions (temperature, malt, nitrogen and oxygen levels) work together to give big differences in yeast growth, and so the chemistry of the final whisky. The more yeast grows, the longer the esters it makes. This means that every whisky really is unique. Chemically. Whisky lactones are ring-shaped esters made from wood acids that leach out of oak barrels during maturation[2]. The number of these depends on how long the whisky is stored, and at what temperatures. They add nutty, woody flavours to the whisky, and accompany tannins. Nobody’s sure how the lactones form, but we suspect that certain molecules can curl up, reacting one of their ends with another, like a snake biting its own tail.


What about the wood?
Wood profoundly influences the flavour of whisky. All sorts of wood components, including lignins, tannins and aldehydes leach out of the oak in contact with alcohol, changing its chemistry.
Lignin, one of the main components of wood, is a polymer that inhabits the cell walls of plants and makes the fibrous backbone or rigid structure of wood. It's what makes wood so good for building things, and helps it to burn slowly to release energy in a controlled way. Lignins in the barrel can degrade on contact with alcohol and get into the whisky, but most of the flavour potential of lignins comes from when it’s burnt, breaking it down into phenols. Lignin itself is pretty tasteless; but phenols, being smaller and more volatile molecules, give distinct aromas. Phenols can be synthesised by yeast, but most enter whisky from contact with burning wood – or peat. Peat is deliberately introduced to whisky to produce the smoky, tangy, dark and bitter flavours that divide whisky tasters. This happens at the drying stage, right at the start of whisky making: the barley used to make whisky is first soaked in water, where it starts to germinate and make sugars ripe for fermentation, then it is dried under hot air or the smoke from burning peat. Peat is common in the boglands of Scotland, and thus the smoky flavour in many whiskies is well cemented into whisky-making tradition.
Lignin is also an immensely complex heteropolymer – one that it’s made up of lots of different bits interconnected and tangled together. Because of its complexity, scientists don’t yet know how it forms in biology, that is, it’s biosynthetic pathway. Understanding how nature regulates and controls the formation of large, complex, yet crucial building blocks such as lignin could considerably broaden our understanding of biochemistry, helping us to create ways in which we can mimic nature to make complicated materials reliably in energetically sustainable ways.


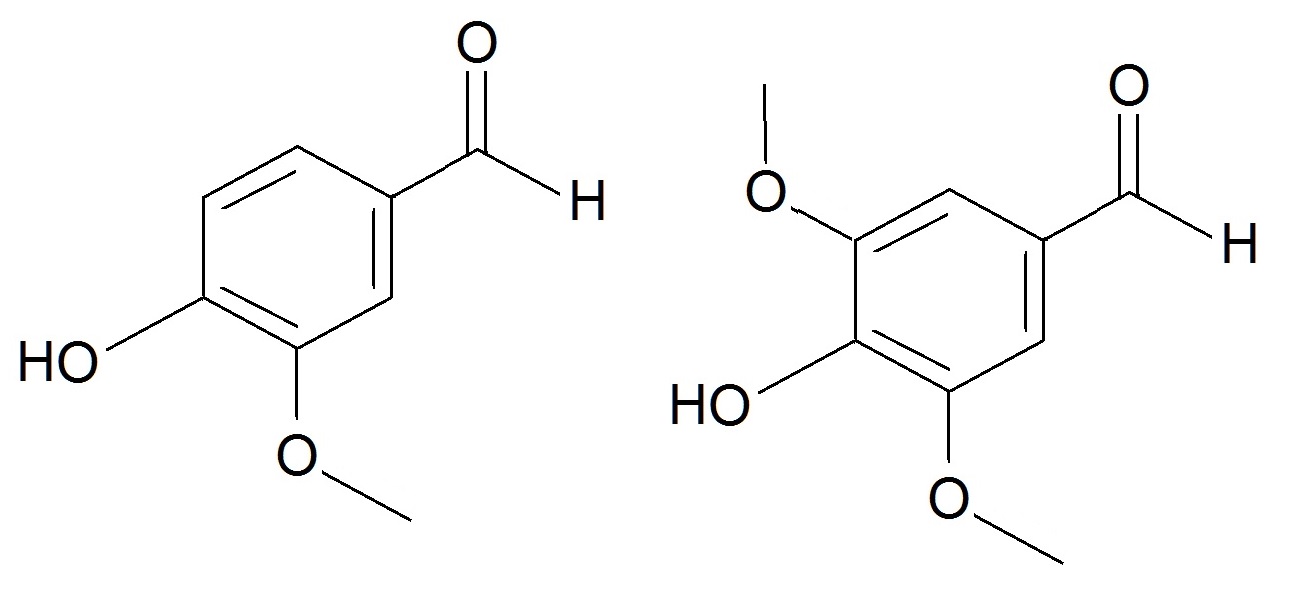
Tannins play a somewhat different role in the tree: instead of providing structure, tannins are bioactive, containing lots of alcohol groups to bind to proteins and so protect a tree from insects, disease and fire. They also promote oxidation of unpleasant sulfurous compounds and alcohols (encouraging the formation of flavour esters), degrade lignin, and provide the whisky with colour. When fruit ripens, the concentrations of tannins drop off, making it less bitter and more appealing, and so indicating that it’s ready to be eaten… or drunk. Tannins are mostly phenols, but the polymer structure of lignin contains things other than phenols, such as vanillins, sugars and acids, all of which are possible degradation products, made when the polymer reacts and splits up. Vanillins are aldehydes. Aldehydes, like esters, are volatile and contribute flavours from the vanilla notes of the vanillins, to the grassy smell of hexanal, to the almond flavour reminiscent of grains and cereals from furfural. They’re commonly found in plants, which means some can come from the original barley from which the whisky’s made, as well as the barrels in which it’s aged. However, aldehydes are fragile under heating and react to form other compounds, so most of the aldehydes probably come from the barrels. One particularly interesting phenol aldehyde is syringaldehyde, which contributes a spicy, woody note to whisky. It also comes from the breakdown of lignin and looks a lot like vanillin. However, it is chemically distinct, and as such its absence can be used to identify counterfeit or imitation whiskies. Some whisky manufacturers use caramel E150A colouring to give their whiskies that desirable colour that usually appears during long aging periods. Some even add vanilla flavourings. Counterfeit Scotch whiskies tend to add both of these, because their whiskies have been produced in ways that aren’t permitted if you want to use the name Scotch whisky – cheaper ways that cut more corners. Although syringaldehyde is commercially available, it’s not as easily accessed as vanillin, and so it doesn’t get added in to counterfeit beverages – making it an excellent chemical fingerprint for quality. Scientists assess whether a whisky is a counterfeit whisky or not using ultraviolet spectroscopy. When ultraviolet light is shone through a small sample tube of whisky, characteristic absorption bands indicate the presence of various chemical species. One of these is syringaldehyde, which appears as a band at 362 nm, a little higher than its cousin vanillin[3].


But one chemical alone can never explain the full complexity of whisky… Other stuff (which will perhaps become more important when imitation whisky-makers start using syringaldehyde extracts) sneaks its way in. Use sherry barrels, and you’ll get chemicals from the sherry that were previously absorbed into the wood released back out. Likewise, older barrels have flavours from previous whiskies on it, and have already leached many of their chemicals, so give a milder, smoother spirit. Temperature and salinity of the storage environment will influence what chemicals are released and how they react together, salt can even penetrate the cask. As a result, whiskies that were made near the coast tend to taste like the coast. Of course, the minerals found in water also influence the chemistry and so flavour of whisky. Streams high in magnesium or iron give very distinct flavours, for example, as well as supplying high concentrations of these essential minerals. It has long been an element of whisky-making folklore to mention the waters upon which the distillery is laid and big up their contribution to its ultimate success. But how big a part does the water play? With esters, acids, aldehydes, lignins, tannins, phenols and all the rest, it’s hard to believe it has the biggest of impacts... Surely?


 2
2
Still, there’s always copper
Historically, distillation chambers or stills were clay or glass, but now all of them seem to be made of copper. This is partly because it’s malleable and conducts the heat through the whisky really well, but it’s also because of centuries of rumour and belief that it does good things to the whisky... One of the rumours, according to Ben Challen, was the story of Old Pulteney whisky: back in the days when the distillery was first setting up, and they designed and built that essential feature – the still. It was made offsite and transported carefully to the distillery in the Highlands of Scotland, where they tried to take it into the building – but couldn’t. The manufacturers, so the story tells, had made a mistake and ordered a still too tall for the distillery. “Still, never mind.” they said, and they lopped the top off the thing – making a low, squat-looking still pot that led to shorter refluxes, forever after blamed for the “dirty” flavour of the Old Pulteney spirit. It’s also been rumoured that copper stills produce less sulfurous whiskies, acting as a catalyst to convert unpleasant-tasting thiols, sulfides and mercaptans made by bacteria and yeast into less pungent alternatives. Although small amounts of thiols and mercaptans add depth, add too much and you end up with eggy whisky. Could there be something in these old wives tales of whiskies? Copper is truly a mysterious metal: a catalyst in many modern chemical processes (sometimes accidentally), even now its role isn’t always fully understood. Partly this is because trace copper has a habit of sneaking into things, so copper-free experiments are not always as copper-free as imagined, leading to inconsistent results. But there definitely are good results that suggest copper does something. For example, dimethyltrisulphide DMTS, which can be detected by humans in concentrations as low as 0.1 µg/l and tends to turn up in whiskies at ten to sixty times this threshold. Using copper wool can reduce the amount of DMTS, although copper salts increase it[4]. If iron is also introduced, the copper becomes less effective. Copper has also been seen to reduce the production of the ester ethyl carbamate, a genotoxic and carcinogenic compound probably dangerous to consume[5]. However, it is not clear whether this is because of a property inherent to copper itself, or just because dissolved copper II salts are necessary to form ethyl carbamate, such that any way of fixing or removing copper II reduces the amount made[6]. These contradictions between copper salts and copper, along with the fact that copper stills don’t particularly corrode, suggest that surface catalysis is happening (if anything), not copper dissolution. Phenols also seem to turn up less when copper is present, although these are more powerfully influenced by the reflux process. The extent and pace of reflux during distillation depends on how full the still is, any pretreatment, the shape of still and how fast it’s cooled afterwards. A high reflux results from little preheating, tall narrow stills, and fast cooling, giving less DMTS and phenols and more esters and alcohols. A low reflux on the other hand, produces more DMTS and phenols and more esters and alcohols (ester and alcohol production is high at either very high or very low reflux, and lower in the middle). Low refluxes therefore give richer, meatier whiskies, that can have a slight pungency.



The differences between beer and whisky
The flavour of whisky, as we have seen, can be affected by lots of things; from water to temperature and humidity, to when, how and what kind of yeast is added – and distilleries only have so much control. As Ben said, I don’t think we’re that good at chemistry yet
. In addition, the market pressure to make reliable products with a distinct flavour customers can associate with your brand and ensure an income stream has made distillers cautious, preferring to stick with traditions they know give good results. Perhaps this goes some way toward explaining why few distilleries experiment with their whiskies – why Glenfiddich always mix their two stills in the same ratio, and why Mortlach, one of the most adventurous, still only do that by changing the ratios of their standard stills in any mix.
But brewers experiment. Lots.
Beer is made from grain like whisky, but it gets its antibacterial properties from hops, whereas whisky undergoes distillation. Most whisky-makers use the same kind of distillers’ yeast, which produces high alcohol, but less flavoursome results than the less alcoholic brewers’ yeast. This also means there is less variation in flavour and greater consistency in whisky than beer. Bacteria also contribute – killed off by the high reflux temperatures used in distillation, cultures are merely thinned and not killed during beermaking, contributing to its flavour and variation. Brewing is also a faster process than whisky-making, making it easier and less costly to experiment. However, these experiments arguably mean we now know a lot more about beers than we do about whisky.
Not that beers have not opted for consistency. Under the Reinheitsgebot or “purity law”, the ingredients of German beers are restricted to water, hops and malt, and methodologies limited, providing consistency in beer-making across breweries throughout Germany (although it must be admitted that this order was originally introduced in Bavaria in 1516 to protect wheat and rye prices for bread-making).
Gaining extra knowledge about the chemical nature of whisky could be helpful. For example, if we knew more about what happened during its production, we could get rid of some of the bad stuff that turns up in whisky. Some of the bad things in whisky are called fusel alcohols – this is literally just German for "bad alcohols". They’re toxic in large quantities. In the small quantities found in whiskies, however, it’s possible they might contribute to hangovers. We don’t know. What we do know is that these are just the "wrong length" alcohols: too long or too short – and they make us sick (although alcohol poisoning from ethanol is still a very real thing).
Learn more about Hangovers.


 2
2However, unless you use distilled water, chemically inert maturation chambers and stills, kill all the bacteria and only add controlled strains, dry the grain under hot air not peat smoke, and a whole load of other things that will destroy the beauty and variety of whiskies, we can’t standardise it – and may never understand it well enough to predict results. ...but then what we made wouldn’t be whisky, would it?
This article was written by the Things We Don’t Know editorial team, with contributions from Rowena Fletcher-Wood.
This article was first published on 2020-11-16 and was last updated on 2020-11-16.
References
why don’t all references have links?
[1] Simpson et al. "Characterization of lactobacilli from Scotch Malt Whisky Distilleries…" Microbiology (2001) 1477, 1007-1016
[2] Tanaka, T. and Kouno, I., 1996. "Whisky lactone precursors from the wood of Platycarya strobilacea." Journal of Natural Products, 59(10), pp.997-999
[3] Panossian, A., et al. "Analysis of aromatic aldehydes in brandy and wine by high-performance capillary electrophoresis." Analytical chemistry 73.17 (2001): 4379-4383.
[4] Harrison, Barry, et al. "The impact of copper in different parts of malt whisky pot stills on new make spirit composition and aroma." Journal of the Institute of Brewing 117.1 (2011): 106-112
[5] World Health Organization’s International Agency for Research on Cancer (IARC) 2007
[6] Riffkin, Harry L., et al. "Ethyl carbamate formation in the production of pot still whisky." Journal of the Institute of Brewing 95.2 (1989): 115-119
Blog posts about whisky

Recent whisky News
Get customised news updates on your homepage by subscribing to articles