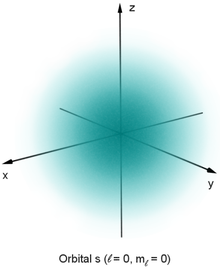
Electrons
Article curated by Rowena Fletcher-Wood
For subatomic particles, electrons are pretty well understood. But we still don’t know what they are, where they are, nor how they spin. Or even, dare I say it, what spin is.
Heisenberg Uncertainty Principle


 2
2Electron Size
A consequence of the cloud-like smeared-outness of the electron, it can’t be contained and measured, making determining electron size complicated. Whilst we know from which cavities it can travel through, e.g. in material structures, that the electron has a “size” or cross-sectional radius, we can’t measure it exactly and know for sure.


Atomic Size
The electron cloud also makes it hard to know how big atoms are – afterall, they’re mostly space with a very tiny nucleus, so how far outwards the electrons travel ends up defining their "edges". But the electron probability distribution or cloud gets thinner further out. Where do you draw the line? Scientists nominally pick a number, say 90% electron density, and call this the size of the atom. But atoms can also change their size and shape as they bond and interact with other atoms. Only isolated gas atoms are easy to estimate the size of: they have no charge and no surroundings, so they remain spherical.
Learn more about How Big Are Atoms?.


Observer effect
Often confused with the Heisenberg Uncertainty Principle is the Observer Effect – the bit of quantum mechanics that states that measuring a quantum object changes its behaviour. The most famous example of this is the Young’s double slit experiment. In this experiment, one particle is fired at a pair of slits and is observed to go through both at once, creating an interference pattern on the other side! This is what you'd expect a wave to do, but not a particle. However, if we get close enough to watch the electron, we see a single particle making a probability-driven decision. Weird.

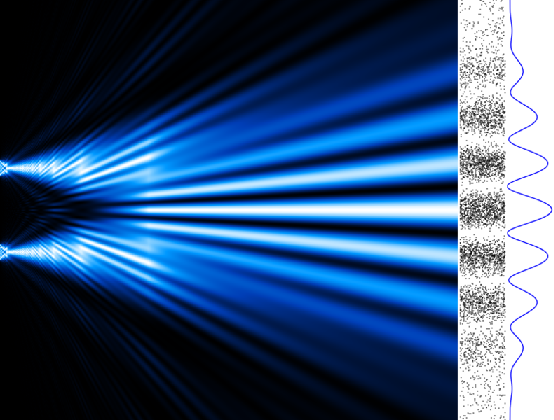
Electron Collisions in an Atom
Another strange thing about the way electrons behave is that they don’t seem to run into each other inside an atom, scatter, lose energy, and destabilise that atom. At least not as far as we’ve observed. Which is a bit odd, whether you see it as a wave or a particle. Not only do we not have a physical excuse for why it can’t happen, but based on probability it should happen, if rarely, since electrons have a non-zero cross-section and, although most of the atom is empty space, we’ve seen them interacting and scattering with other things, like x-rays. So why not each other? Perhaps this is something we don’t yet understand to do with the Pauli Exclusion Principle, somehow prohibiting electrons with different sets of quantum numbers from interacting.


Pauli Exclusion Principle
According to the Pauli Exclusion Principle, no two electrons can be described by the same four quantum numbers. This means they have to be distinguishable from each other: they can’t look the same and occupy exactly the same space. There no clear reason in our current electron models (waves or particles) for two electrons to be distinguishable, and the rule only applies to fermions like the electron, not to boson particles like the photon, which cluster and behave quite differently, producing effects like superconductivity. Indeed, the Pauli Exclusion Principle is an observation that tells us about how electrons behave, but leaves us scratching our heads as to why.


 2
2
Electron Spin
Spin is a property of angular momentum intrinsic to particles, and describes the way they are deflected by magnetic fields. That is, subatomic particles like electrons act as if they were spinning rapidly to generate tiny magnetic fields. The property was originally named spin based on this observation. However, it doesn't literally describe spinning particles[2]. So what is it? We don’t know. It’s a physical property, and it’s quantised, meaning only certain spins (up and down) are allowed. It also extends to other systems beyond the electron, such as isotopic nuclear spin, and “up” and “down” quarks. What we do know is that it can’t be a literal spin. The concept that electrons really rotate breaks down under the watchful eye of quantum mechanics. For example, to make the observed magnetic fields, the surface of the electron would have to be spinning faster than the speed of light (and that is not allowed!).


Is our very well known particle the electron actually spinning out of control?
This article was written by the Things We Don’t Know editorial team, with contributions from Johanna Blee, and Rowena Fletcher-Wood.
This article was first published on 2021-05-24 and was last updated on 2021-05-24.
Recent electrons News
Get customised news updates on your homepage by subscribing to articles